

Waste tantalum recovery source
Tantalum resources are scarce and expensive, and the utilization of secondary resources has special significance. The secondary resources of niobium and tantalum include two parts: one is the waste generated during the smelting and processing of tantalum and niobium, and the other is the scrapped components of tantalum and niobium products during use. At present, tantalum recovered from secondary resources accounts for about 15% to 20% of the tantalum raw material amount.
According to the form of waste, tantalum niobium waste mainly includes three categories: pure metals, compounds, and alloys. Pure metal waste is generally recovered through furnace smelting methods such as vacuum melting, electron beam melting, and hydrogenation powder production after chemical cleaning. There are various types and complex compositions of waste materials such as compounds and alloys, for which various recycling processes have been developed.
Recovery of hard alloys containing tantalum
Tantalum containing hard alloy is an alloy composed of tungsten carbide as the basic composite carbide (WC-TiC-TaC-NbC) and titanium cobalt, with a complex composition and low tantalum niobium content. It is generally only recovered as an enrichment material.
1. Zinc treatment method
The sintered carbide is first decomposed with liquid zinc at 800 ℃ to break the bonding bond between the carbide particles and the metal titanium cobalt. The decomposed product is then separated from zinc by vacuum distillation and recycled. The product after zinc removal is finely ground and oxidized, followed by alkaline treatment and water leaching. Tungsten enters the leaching solution in the form of NaWO3 (from which ammonium paratungstate is produced). The tungsten removal residue is then leached with sulfuric acid for cobalt and titanium (further separation and recovery of cobalt and titanium from the sulfuric acid solution), and the leaching residue is the tantalum niobium enrichment material. The process flow of this method is shown in the following figure:
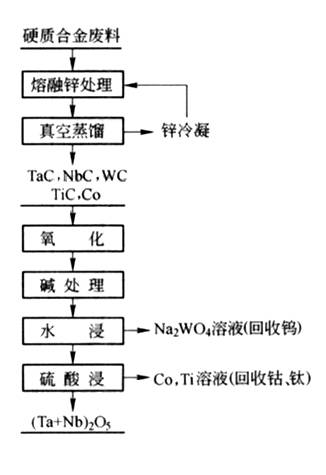
2. Sodium nitrate melt enrichment method
Hard alloy waste is first melted together with sodium nitrate at 700-800 ℃ to decompose and oxidize the hard alloy carbides. The obtained molten material is first leached with water (followed by tungsten extraction), and the filtered residue is leached with hydrochloric acid for cobalt (followed by cobalt recovery). Tantalum and niobium are enriched in the hydrochloric acid leaching residue. The obtained enrichment material contains Ta2O530.4%, WO31.26%,TiO238.6%。
Tantalum capacitor waste treatment
The recycling of waste tantalum capacitors is quite complex, especially for liquid tantalum capacitors with metal cladding. The outer shell must be first removed by chemical methods (electrolysis, aqua regia acid washing) or mechanical methods, then deoxidized by sodium reduction or carbon reduction, and finally melted by electron beam to obtain tantalum ingots. Resin coated solid tantalum capacitors are first treated with sulfuric acid to remove the plastic casing; For the chip capacitors, they are crushed and the wires are not picked out by magnetic separation. The plastic is separated by re selection method, and the remaining anode blocks are leached out with sodium hydroxide for tin, silver is dissolved in nitric acid, manganese is leached out with hydrochloric acid, deoxygenated by sodium reduction method, and then melted in an electron beam furnace to obtain tantalum ingots. The process flow is shown in the following figure:
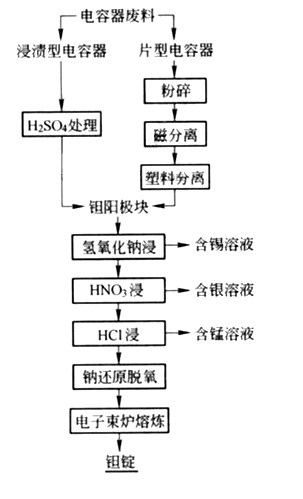
Recovery of waste lithium tantalate
The recovery of waste lithium tantalate mainly includes two types: pyrometallurgical and wet methods. There are mainly aluminum thermal reduction methods in pyrometallurgy, which use aluminum as a reducing agent to reduce single crystal powders to niobium iron or metallic tantalum or niobium, and then melt them in an electron beam furnace to obtain pure tantalum or niobium. Wet methods mainly include alkali treatment, and the process flow is shown in the figure:
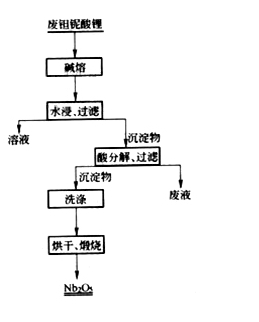
After crushing the waste lithium tantalate single crystal, it is melted with sodium hydroxide at 700-800 ℃, and the fusion reaction is as follows:
2LiTaO3+10NaOH=2Na2TaO5+Li2O+5H2O
Melt undergoes hydrolysis treatment to convert Na2TaO5 into Na2O • Ta2O5 hydrate:
6Na5TaO5+36H2O=4Na2O•3Ta2O5•25H2O+22NaOH
The precipitate was treated with 6mol/L hydrochloric acid to convert tantalum hydrate into Ta2O5 • xH2O, and lithium was separated from tantalum by generating LiCl.
4Na2O•3Ta2O5+8HCl+(x-7)H2O=3Ta2O5•xH2O+8NaCl
Obtain Ta2O5 with a purity of 98%. This method can simultaneously recover lithium hydroxide.